Science & Technology
January 27, 2017 · 16 comments
16 comments
The hydrogen was subjected to immense pressures. Image Credit: R. Dias and I.F. Silvera
It's a result that was predicted over 80 years ago by Eugene Wigner and Hillard Bell Huntington, two scientists who determined that at sufficient enough pressures it was possible to turn hydrogen in to a metal - something that was impossible to prove at the time.
"If this experiment is reproducible, it solves experimentally one of the major outstanding problems in all of physics," said Jeffrey McMahon from Washington State University.
The scientists behind the achievement now predict that metallic hydrogen, once created, may actually remain in either a solid or liquid state even at normal temperatures and pressures.
It is so dense in fact that it could even prove to be an effective replacement for rocket fuel.
Source: New Scientist | Comments (16)
Metallic hydrogen created for the first time
By T.K. RandallJanuary 27, 2017 ·
 16 comments
16 comments
The hydrogen was subjected to immense pressures. Image Credit: R. Dias and I.F. Silvera
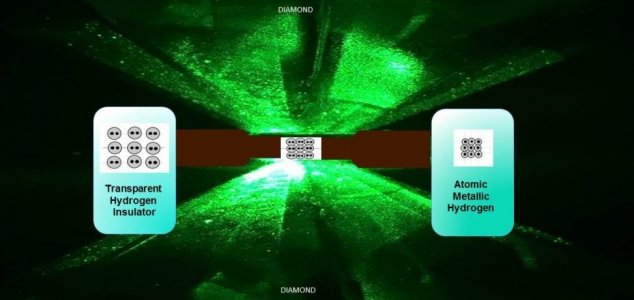
Scientists have succeeded in squeezing hydrogen to such an extreme that is has turned in to a metal.
The remarkable breakthrough was made by two researchers at Harvard University who used diamonds to squeeze a hydrogen sample to pressures greater than those at the Earth's core.It's a result that was predicted over 80 years ago by Eugene Wigner and Hillard Bell Huntington, two scientists who determined that at sufficient enough pressures it was possible to turn hydrogen in to a metal - something that was impossible to prove at the time.
The scientists behind the achievement now predict that metallic hydrogen, once created, may actually remain in either a solid or liquid state even at normal temperatures and pressures.
It is so dense in fact that it could even prove to be an effective replacement for rocket fuel.
Source: New Scientist | Comments (16)

The Unexplained Mysteries
Book of Weird News
AVAILABLE NOW
Take a walk on the weird side with this compilation of some of the weirdest stories ever to grace the pages of a newspaper.
Click here to learn more

Support us on Patreon
BONUS CONTENTFor less than the cost of a cup of coffee, you can gain access to a wide range of exclusive perks including our popular 'Lost Ghost Stories' series.
Click here to learn more
United States and the Americas
Ancient Mysteries and Alternative History
Spirituality, Religion and Beliefs
Extraterrestrial Life and The UFO Phenomenon
Total Posts: 7,734,587 Topics: 322,944 Members: 202,892
Not a member yet ? Click here to join - registration is free and only takes a moment!
Not a member yet ? Click here to join - registration is free and only takes a moment!
























Please Login or Register to post a comment.